Re: Seasonal Flu 2008 - 2009
The level of antiviral resistance is actually near 100% in H1N1 and H3N2. In H3N2 the resistance is to the amantadines and is primarily S31N. This change is also in clade 2C of H1N1.
For Tamiflu (oseltamivir) the level is 100% in clade 2B.
Thus, in Europe and North America virtually all seasonal flu has antiviral resistance.
H274Y in H1N1 likely came from H5N1, but after showing up in clade 2C in China, it jumped from clade to clade via recombination, before hitchhiking with the dominant H1N1 sub-clade, which is now dominant in Europe and North America.
Originally posted by AlaskaDenise
View Post
For Tamiflu (oseltamivir) the level is 100% in clade 2B.
Thus, in Europe and North America virtually all seasonal flu has antiviral resistance.
H274Y in H1N1 likely came from H5N1, but after showing up in clade 2C in China, it jumped from clade to clade via recombination, before hitchhiking with the dominant H1N1 sub-clade, which is now dominant in Europe and North America.
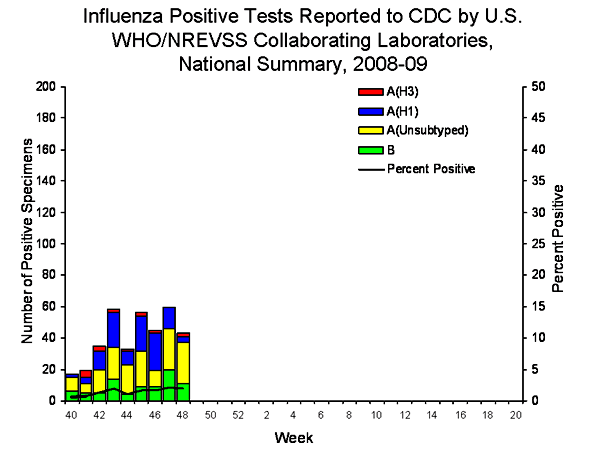
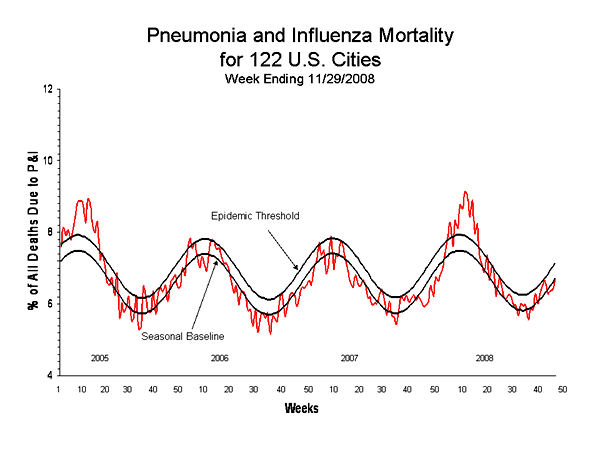
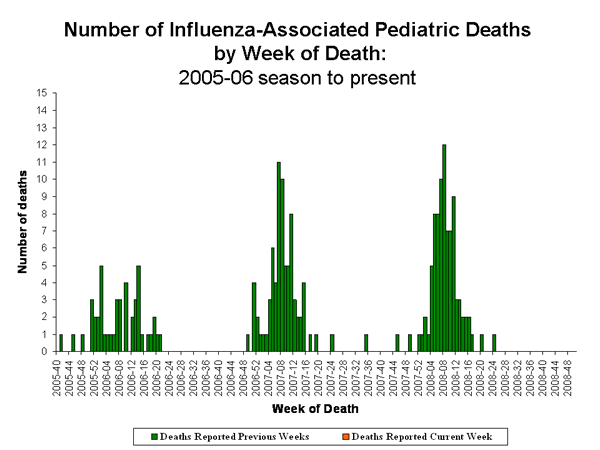
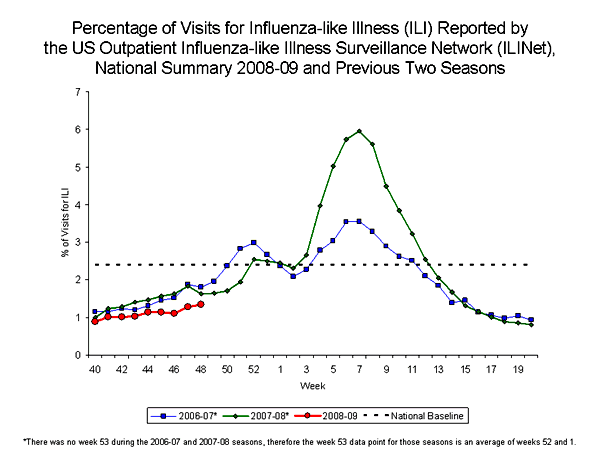





Comment